Point of Care Testing and the Quality Equation
When it comes to evaluating a patient's wellness and determining cause and severity of illness, diagnostics are an essential component of the physician's toolbox. Traditionally, most diagnostic tests are performed in sophisticated laboratories, but with the availability of newer technologies, it is now practical to perform tests in your office rather than sending them to an outside laboratory. By bringing point of care tests (POCT) into your practice, you can ensure that the test is performed, and obtain results during a patient visit. Patients are relieved of the worry that comes with waiting for results, and you can begin to establish a follow-up or treatment plan right then and there.

Point of Care Testing and the Quality Equation
June 15, 2021
.
When it comes to evaluating a patient's wellness and determining cause and severity of illness, diagnostics are an essential component of the physician's toolbox. Traditionally, most diagnostic tests are performed in sophisticated laboratories, but with the availability of newer technologies, it is now practical to perform tests in your office rather than sending them to an outside laboratory.
By bringing point of care tests (POCT) into your practice, you can ensure that the test is performed, and obtain results during a patient visit. Patients are relieved of the worry that comes with waiting for results, and you can begin to establish a follow-up or treatment plan right then and there.
For these reasons, physicians are increasingly considering the implementation of POCT in their practices. But beware, not all POCT are created equal, and one key point of differentiation can be quality.

A Question of Quality
When it comes to POCT, there are many aspects of quality to consider, and a multitude of factors that can affect quality. At its essence, you want to be confident that a system will deliver the right results, consistently and reliably.
A failure to adequately evaluate the quality of a system before implementation, or to follow through on steps to maintain quality can lead to costly errors that could have severe consequences:1
• Misdiagnosis and improper treatment with potentially harmful outcomes
• Missed (no) diagnosis and no treatment when needed
• Repeat testing due to questionable results
• Costly follow-up procedures, including unnecessary surgery
• Worry and anxiety for patients and parents/families
• Additional costs to the healthcare system
In assessing a POCT, you will want to consider the complexity of the test. In general, a POCT will be CLIA waived or non-CLIA waived. CLIA waived tests are considered to be less complex, and are not subject to the same regulatory requirements (personnel, quality control, proficiency testing or other quality assurance) to which moderate complexity laboratories are subjected.1 Operators performing the waived tests are only required to follow the specifications of the assay manufacturer's operating instructions.1
The American Association of Clinical Chemistry (AACC) suggests that analytical accuracy should be part of an assessment framework for POCT.2 Two indicators key to this assessment are precision and accuracy. Precision means that the same sample run multiple times on the same machine gives the same result. When a test is precise, the amount of random variation is small. An indicator of precision is a test's coefficient of variation or CV. This statistic will vary among tests, so you should research the desirable CVs for the tests you are planning to implement.
A test method is said to be accurate when it measures what it is supposed to measure.3 This means it is able to detect and measure the true amount or concentration of a substance in a sample.3 There is no definitive means of assessing accuracy across the board. This can be test-specific. Often, you can find or ask about published papers evaluating the accuracy of a particular test.
For some tests, two other important statistical standards are sensitivity and specificity. Diagnostic specificity and sensitivity reveal the likelihood of false negatives and false positives.3
• Diagnostic Sensitivity measures a test's ability to correctly identify a positive result in a person who has a given disease or disorder. A test with high sensitivity is less likely to generate a false-negative result.
• Diagnostic Specificity measures a test's ability to correctly report a negative result in people who are free of a given disease or disorder. A test with high specificity is less likely to generate a false-positive result.
A variety of websites and online publications provide more detail on statistical evaluation of POCT.
But Wait; There's More!
In addition to statistical measures of quality, you should also be mindful of the type of quality control (QC) process for a device that you might implement. Some tests require that the operator perform QC at regular intervals. Others, such as HemoCue®'s, have built-in QC functions that automatically perform QC every time the device is turned to ensure that the test is working properly. Minimizing manual processes not only saves time, but it can also reduce opportunities for error.
There are many other factors that can affect the quality of in-office testing. An American Association of Family Physicians review of CLIA guidelines points out that quality control for physician office laboratories encompasses the entire testing process: pre-analytical, analytical (testing) and post-analytical.4 This means that ensuring quality can extend to administration, training, personnel, etc. Regardless of whether you are performing tests in an office laboratory or not, it is good practice to address quality throughout the entire continuum of testing.
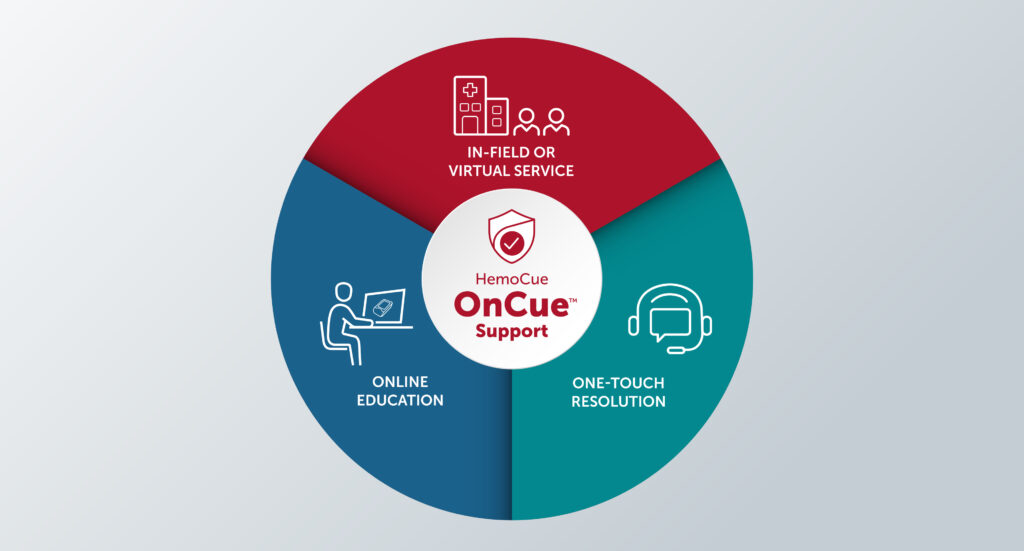
Understanding the type of training and support that a device manufacturer will provide is essential to making a decision. Support-intensive companies such as HemoCue provide comprehensive programs to ensure that customers are fully supported. HemoCue OnCue™ Support offers in-person and virtual technical assistance, product education and training, which ensures that customers can obtain the information they need when they need it.
In addition, HemoCue OnCue™ goes a step further with its One-Touch Resolution commitment to resolving customers' support needs as quickly and in as few steps as possible. Live customer service and technical support experts, based in California, are ready to help:
• Provide answers and in-depth knowledge about HemoCue products.
• Troubleshoot issues in one call-no endless transfers.
• Get status on orders and shipping.
This type of service and support offers a strong advantage in easing your implementation of new technology and ensuring that your practice is prepared to support high-quality testing.
Finally, be sure to research a system's connectivity as this can factor into quality as well. Features to look for include:
• Automated transcription
• Data capture and transmission to electronic health records
• Reporting capabilities
These types of connectivity and information management features can improve patient safety, augment clinical utility, reduce the risk of errors, enhance audit trails and facilitate billing processes.
Undoubtedly, quality matters and should not be taken lightly, but the multitude of considerations can be overwhelming. Luckily, there are resources to help guide the process. Investing the time to research products and processes will ensure that you ask the right questions and lay a good foundation for POCT in your office.
For more information, go to:
Acutecaretesting.org
Quality Assurance CLIA and Your Lab
AACC Guidance on Management of Point of Care Testing
Footnotes:
1 Okorodudu, Anthony O. "Optimizing accuracy and precision for point-of-care tests," Acutecaretesting.org, Apr. 2011, https://acutecaretesting.org/en/articles/optimizing-accuracy-and-precision-for-point-of-care-tests.
2 AACC Academy. "AACC Guidance Document on Management of Point-Of-Care-Testing," 4 Jun. 2020, https://www.aacc.org/science-and-research/aacc-academy-guidance/management-of-point-of-care-testing.
3 "Accuracy, Precision, Specificity and Sensitivity," viewed 27 May 2021, https://www.labtestsonline.org.au/understanding/test-accuracy-and-reliability/how-reliable-is-pathology-testing.
4 "Quality Assurance CLIA and Your Lab," AAFP, viewed 27 May 2021, https://www.aafp.org/family-physician/practice-and-career/managing-your-practice/clia/quality-assurance.html.
Related Articles
Introducing the HemoCue Customer Portal